When the data is unbalanced or there are missing values, repeated measures ANOVAs fail to report unbiased results. Furthermore, all the data of one individual will be ignored if data from one time point is missing. Multi-level linear models gets around this by comparing models of the data, not the data itself, using log-likelihood/Chi squared distribution analyses. Multi-level linear models also avoid sphericity issues. In general multi-level linear models are better in every way to a repeated measures ANVOA.
#Loading data
example<- read.table(url("https://jackauty.com/wp-content/uploads/2023/11/example4.txt"), header=T)
example$ID<-as.factor(example$ID)
#Installing packages (but only if you need to install them)
if(!require(MASS)) install.packages("MASS")
if(!require(sjPlot)) install.packages("sjPlot")
if(!require(ggplot2)) install.packages("ggplot2")
if(!require(emmeans)) install.packages("emmeans")
if(!require(viridis)) install.packages("viridis")
library(ggplot2); library(sjPlot); library(MASS); library(emmeans);
#Running multi-level linear model
install.packages("lme4")
require(lme4)
lme1<-lmer(Dependent~1 + (1|ID), data=example, na.action=na.omit)
lme2<-update(lme1, .~. +Factor1)
lme3<-update(lme2, .~. +Factor2)
lme4<-update(lme3, .~. +Factor1*Factor2)
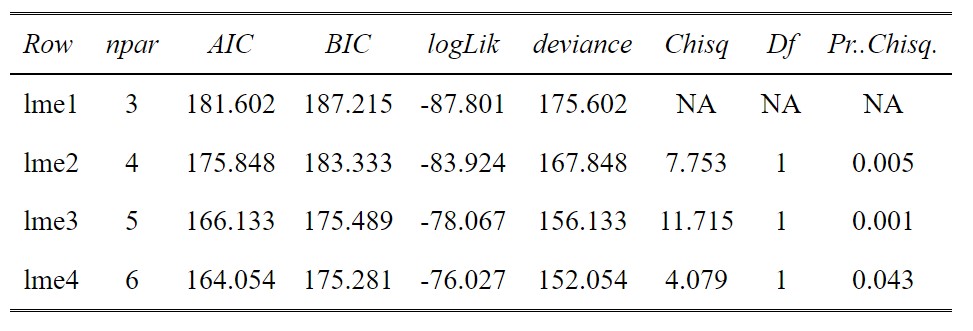
anova(lme1,lme2,lme3,lme4)
Assumption checking
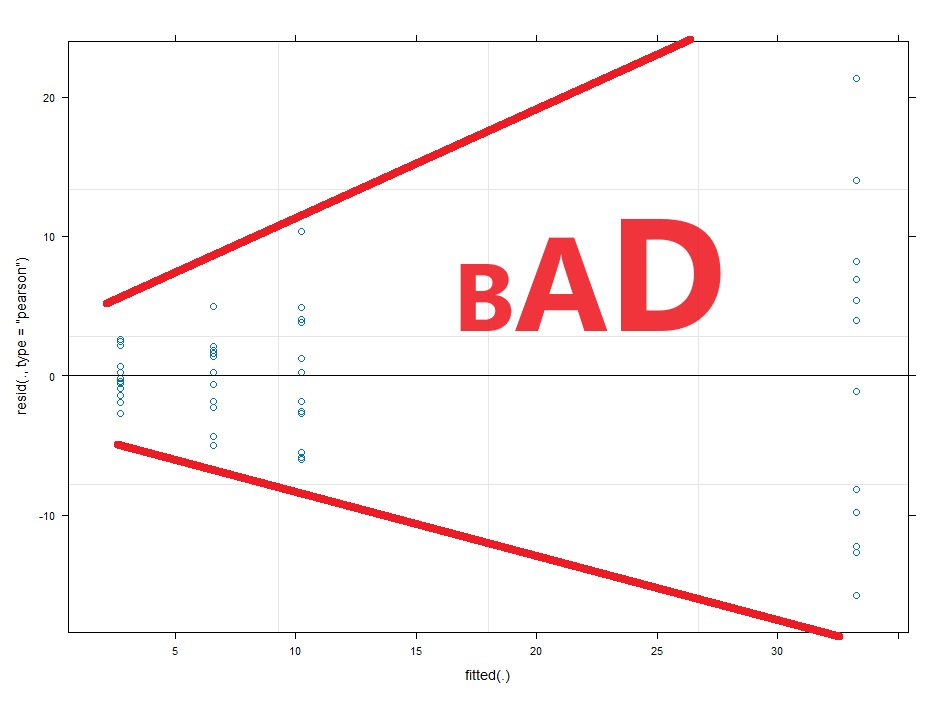
Now with multi-level linear models (AKA repeated measures ANOVA) there are two kinds of residuals. There is the variability of the raw data around the predicted value (this is the conditional residual), and the variability of the random effect (e.g. animal or human). The random effects should be normalish but don’t have to be homoscedastic. The conditional residuals should be both normal and homoscedastic.
#Assumption check of conditional residuals plot(lme4)
#Now we check for normality of the conditional residuals
qqnorm(resid(lme4))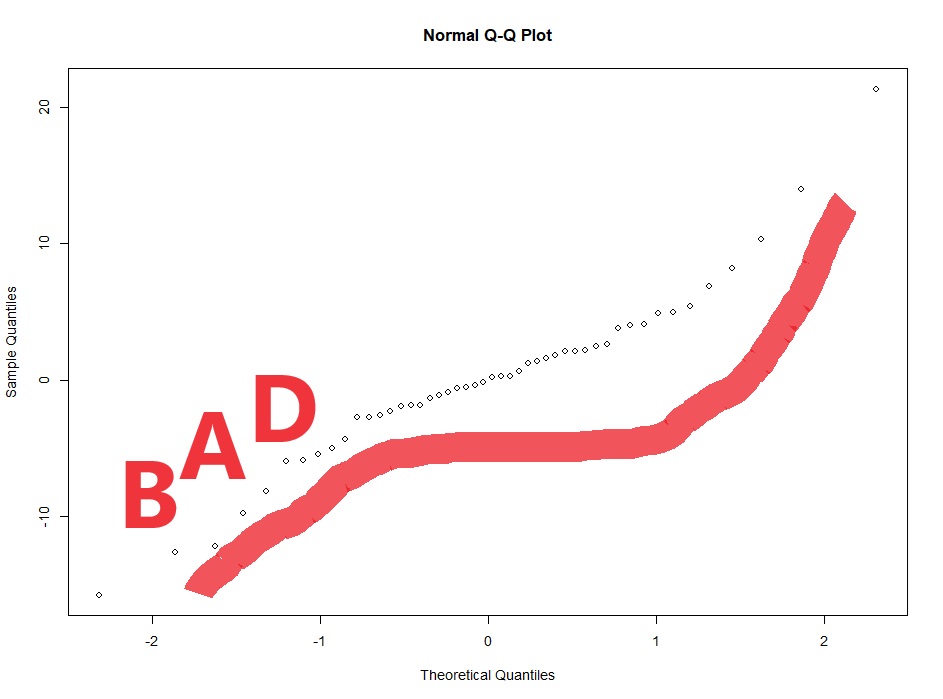
# checking the normality of the random effects (here random intercept):
qqnorm(ranef(lme4)$ID$'(Intercept)',
main="Q-Q plot for the random intercept")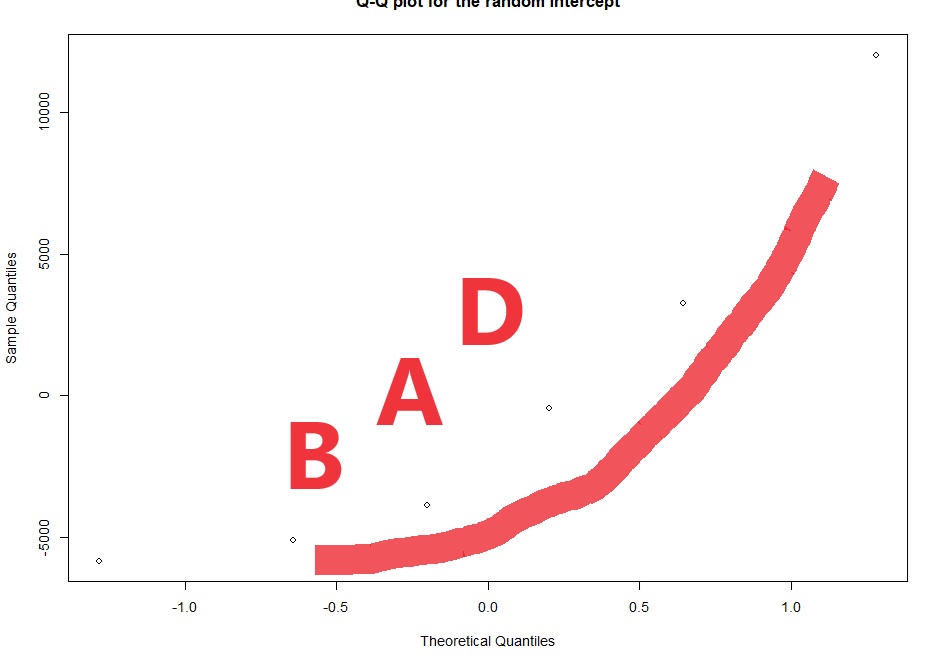
Transforming the data
So you can just have a guess at a transformation needed. The wedge shaped residual vs fitted plot above tells us we need a shrinking transformation like a LOG10 or square root. However, if we can take the researcher out of the process this is better. What if the LOG10 and square root have similar diagnostic plots, but the LOG10 gives you significant results and the square root transformation gives you p=0.051. You might be tempted to choose the LOG10 transformation because it gives you significance.
What we can do is a BoxCox transformation. This is an automated process that transforms your data by a range of powers and evaluates the normality of the residuals. Then it chooses the power (Selected.lamda) that produces the most normally distributed residuals. Typically, when you solve normality, you also solve homoscedasticity.
Now you can do this on a multi-level linear model, but often fixing a simple model will also fix the complex model. So we’ll run a simple linear model, calculate what we should transform the data by, then apply that transformation to our multi-level linear model.
#Making the simple linear model
simple.linear<-lm(Dependent~Factor1*Factor2, data=example)
#BoxCox transformation
boxcox<-boxcox(simple.linear,lambda = seq(-5, 5, 1/1000), plotit = TRUE )
Selected.lambda <-boxcox$x[boxcox$y==max(boxcox$y)]
Selected.lambda
#Run the transformed model
lme1<-lmer((Dependent+1)^Selected.lambda~1 + (1|ID), data=example, na.action=na.omit)
lme2<-update(lme1, .~. +Factor1)
lme3<-update(lme2, .~. +Factor2)
lme4<-update(lme3, .~. +Factor1*Factor2)
anova(lme1,lme2,lme3,lme4)
#Assumption check
plot(lme4)
qqnorm(resid(lme4))
# checking the normality of the random effects (here random intercept):
qqnorm(ranef(lme4)$ID$'(Intercept)',
main="Q-Q plot for the random intercept")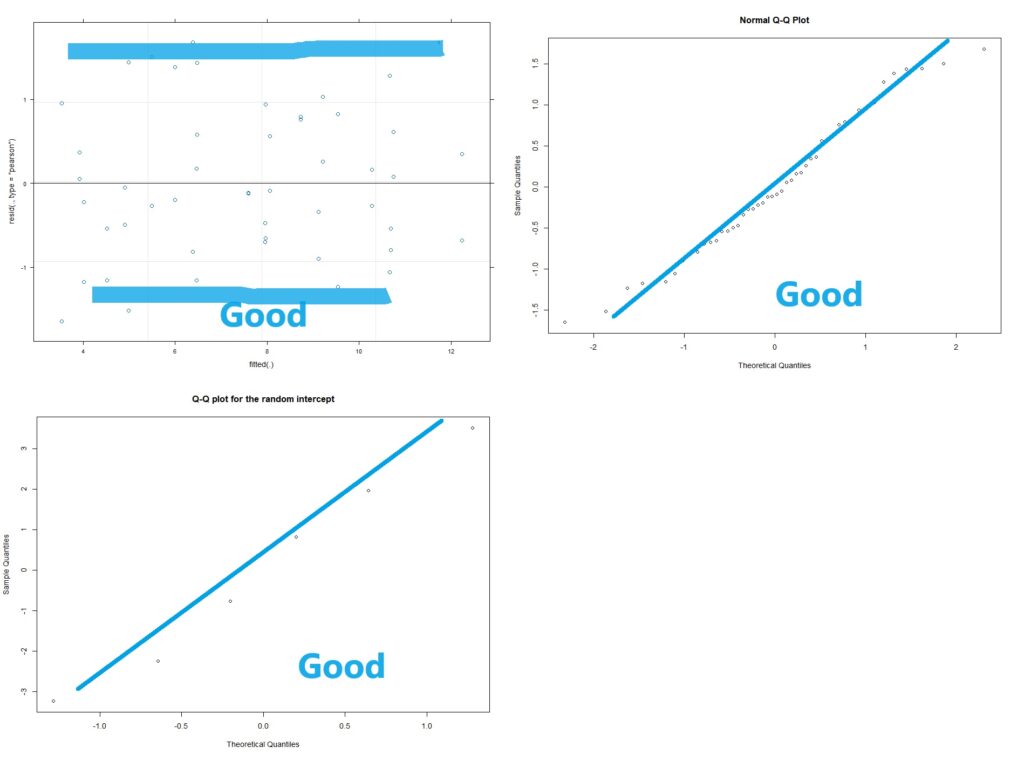
Posthocs!
OK this is a Post-hoc analysis with a priori selected comparisons. Perhaps we don’t want to do all the comparisons, because some are useless. For example, why compare Females on Drug with Males on Placebo? That doesn’t make sense. So in this, we select our comparisons and adjust for only those comparisons.
#Posthoc/Planned contrasts
post <- emmeans(lme4, pairwise ~ Factor1*Factor2, adjust="none")
posthoc<-summary(post)
#Look at the table of potential comparisons
tab_df(posthoc$contrasts[,c(1,4)],show.rownames =T)
#Select the comparison you are interested in but adding the row numbers below
comparisons<-c(1,2,5,6)
#Build a dataframe of all the comparisons (contrasts) and the unadjusted p values
p.value<-data.frame(cbind(posthoc$contrasts["contrast"],posthoc$contrasts["p.value"]))
ptable<-p.value[comparisons,]
#Create a column of adjusted p values using the Holm-Sidak method
ptable$padj<-p.adjust(ptable$p.value, method="holm")
#View the table in a pretty way
tab_df(ptable,digits = 4) 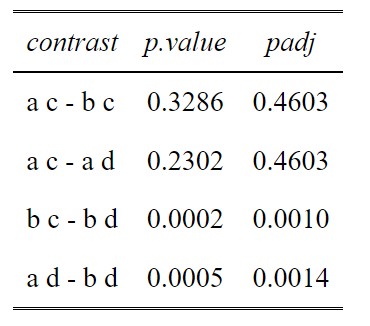
How I would publish the above method.
Linear mixed modelling was used to evaluate the effect of explanatory variables on the dependent variable (package lme4, Bates et al. 2015). All factors and interactions were modelled as fixed effects. A within-subject design with random intercepts were used for all models. The significance of inclusion of an independent variable or interaction terms were evaluated using log-likelihood ratio. Holm-Sidak post-hocs were then performed for pair-wise comparisons using the least square means (package “emmeans”, Lenth 2023). Homoskedasticity and normality of the Pearson residuals were evaluated graphically using predicted vs residual and Q-Q plots, respectively, and Box Cox transformations were applied when necessary (package “MASS” Venables 2002). All analyses were performed using R (version 4.3.3).
#Making a reference list
pkg <- c("MASS","sjPlot","lme4","emmeans")
#Create a loop to build a dataframe of the references
citation_list<-factor()
for(i in pkg){ citation_list<- c(citation_list,paste(capture.output(print(citation(i), style ="text",bibtext=F)), collapse="\n")) }
#Display the dataframe nicely with sjPlot's tab_df
tab_df( data.frame(citation_list), col.header="References", sep=" ") | Venables WN, Ripley BD (2002). _Modern Applied Statistics with S_, Fourth edition. Springer, New York. ISBN 0-387-95457-0, . |
| Lüdecke D (2023). _sjPlot: Data Visualization for Statistics in Social Science_. R package version 2.8.14, . |
| Wickham H (2016). _ggplot2: Elegant Graphics for Data Analysis_. Springer-Verlag New York. ISBN 978-3-319-24277-4, . |
| Lenth R (2023). _emmeans: Estimated Marginal Means, aka Least-Squares Means_. R package version 1.8.6, . |
| Bates D, Mächler M, Bolker B, Walker S (2015). “Fitting Linear Mixed-Effects Models Using lme4.” _Journal of Statistical Software_, *67*(1), 1-48. doi:10.18637/jss.v067.i01 . |
All the code at once! (easier to copy)
#Installing packages (but only if you need to install them)
if(!require(MASS)) install.packages("MASS")
if(!require(sjPlot)) install.packages("sjPlot")
if(!require(ggplot2)) install.packages("ggplot2")
if(!require(emmeans)) install.packages("emmeans")
if(!require(viridis)) install.packages("viridis")
library(ggplot2); library(sjPlot); library(MASS); library(emmeans);
#Loading data
example<- read.table(url("https://jackauty.com/wp-content/uploads/2023/11/example4.txt"), header=T)
example$ID<-as.factor(example$ID)
#Running multi-level linear model
lme1<-lmer(Dependent~1 + (1|ID), data=example, na.action=na.omit)
lme2<-update(lme1, .~. +Factor1)
lme3<-update(lme2, .~. +Factor2)
lme4<-update(lme3, .~. +Factor1*Factor2)
anova(lme1,lme2,lme3,lme4)
#Assumption check
plot(lme4)
qqnorm(resid(lme4))
# checking the normality of the random effects (here random intercept):
qqnorm(ranef(lme4)$ID$'(Intercept)',
main="Q-Q plot for the random intercept")
simple.linear<-lm(Dependent~Factor1*Factor2, data=example)
#BoxCox transformation
boxcox<-boxcox(simple.linear,lambda = seq(-5, 5, 1/1000), plotit = TRUE )
Selected.lambda <-boxcox$x[boxcox$y==max(boxcox$y)]
Selected.lambda
#Run the transformed model
lme1<-lmer((Dependent+1)^Selected.lambda~1 + (1|ID), data=example, na.action=na.omit)
lme2<-update(lme1, .~. +Factor1)
lme3<-update(lme2, .~. +Factor2)
lme4<-update(lme3, .~. +Factor1*Factor2)
tab_df(anova(lme1,lme2,lme3,lme4),show.rownames =T, digits=3)
#Assumption check
plot(lme4)
qqnorm(resid(lme4))
# checking the normality of the random effects (here random intercept):
qqnorm(ranef(lme4)$ID$'(Intercept)',
main="Q-Q plot for the random intercept")
#Create a linear model and do a Box-Cox transformation
#Posthoc/Planned contrasts
#Posthoc/Planned contrasts
post <- emmeans(lme4, pairwise ~ Factor1*Factor2, adjust="none")
posthoc<-summary(post)
tab_df(posthoc$contrasts[,c(1,4)],show.rownames =T)
##Now select the rows of the comparisons you are interested in
comparisons<-c(1,2,4)
#Build a dataframe of all the comparisons (contrasts) and the unadjusted p values
p.value<-data.frame(cbind(posthoc$contrasts["contrast"],posthoc$contrasts["p.value"]))
#Select the comparison you are interested in
comparisons<-c(1,2,5,6)
ptable<-p.value[comparisons,]
#Create a column of adjusted p values using the Holm-Sidak method
ptable$padj<-p.adjust(ptable$p.value, method="holm")
#View the table in a pretty way
tab_df(ptable,digits = 4)
#Making a reference list
pkg <- c("MASS","sjPlot","lme4","emmeans")
#Create a loop to build a dataframe of the references
citation_list<-factor()
for(i in pkg){ citation_list<- c(citation_list,paste(capture.output(print(citation(i), style ="text",bibtext=F)), collapse="\n")) }
#Display the dataframe nicely with sjPlot's tab_df
tab_df( data.frame(citation_list), col.header="References", sep=" ")
Hi Jack,
Thanks this is really helpful.
Quick question on assumptions of normality/spherecity. After you’ve fitted the model and plotted the residuals. If they don’t follow a normal distribution, is it still ok to interpret the ouput of the model and perform post-hoc testing? I myself have a rather complex experiment to analyse (3 way repeated measures) and want to make sure I properly analyse the data.
Thanks,
Dave
Hi David,
Great question. The first thing I would do is try to transform your data in such a way that the residuals become normally distributed. Often with biological data you have to log or square-root the data. Check out this post https://jackauty.com/anovas-one-way-and-two-way/, it goes into more detail around the assumptions of general linear models and to transform your data. However, there are times when this won’t work. Particularly if your data is very discrete, like a score out of 10. In this case you’ll need to look into generalized linear modeling, information on this can be found here https://jackauty.com/generalized-mixed-modelling/. If these options fail, your last hope is a non-parametric test. This still assumes equal variance, but it doesn’t assume anything about the distribution (e.g. normally distributed). However, non-parametric tests can have very low power in some situations, meaning a substantial difference may not come up significant. So I would plan on using the other options first.
Hi Jack,
This method has been tremendously useful specifically for a rather tricky dataset I am working with. I have a two-factor repeated measures design with unbalanced data (between 10-20 reps). One factor has 4 levels, the other has 2. My best model based on maximum parsimony is the model with both factors included, but without the interaction term (e.g., lme3 in your example; my lme4 had a non-significant p-value). I have 16 posthoc comparisons of interest. When I run the posthoc, I couldn’t help but notice a lot of the unadjusted AND adjusted p-values are identical out to a ridiculous number of decimal places (similar to your example results between a,c – a,d and b,c – a,d). My questions is “why is this happening?”
The means and variances of each of the factor levels are clearly different when I tabulate them. What is happening “under the hood” that could be causing identical numbers to that many decimal places?
Thanks,
Edwin
Hi Edwin,
I’d love to see your code for this. My guess is that your model doesn’t actually have the complexity to do 16 comparisons in your post-hoc. If your variables don’t interact, then only some post-hocs actually make sense to be performed.
Think about this:
If your model says that men and women are effected by a drug the same (e.g. effect ~ sex + drug), then your model would predict the exact same difference between men-placebo vs men-drug as women-placebo vs women-drug. So you would get the exact same p-value.
Now, the concept of post-hocs is complicated. Post-hocs were originally designed for probing data agnostic to a hypothesis. Obviously, this isn’t how experiments are run. We have hypotheses and, when we plan the experiments to test these hypotheses, we have specific comparisons in mind. These are sometimes called a priori contrasts, or planned contrasts. Mathematically, there is no difference between a post-hoc and planned contrast, except, some people argue you don’t have to adjust for multiple comparisons with planned contrast (I disagree, as would most peer reviewers). One slight difference, is that you can select just a few comparisons with planned contrasts and thus reduces the price you pay when you adjust for multiple comparisons. Anyway all of this is to say, if you were always going to compare these groups and this was the plan from the beginning, you are totally justified in putting the full model in (dependent ~ explanatory 1 * explanatory 2) to generate your estimates for your post-hocs (planned contrasts).
Hope this helps.